Chemistry
A collection of Chemistry related resources
curated by Benetech
Resources 67
-

Acids and bases are important to many chemical processes: maintaining a stable internal environment in the human body, baking a delicious cake, or determining whether a lake can support aquatic life. Reactions involving acids and bases can be described through the transfer of protons. The reactions of acids and bases, which can be monitored with indicators, can range from corrosive behavior to neutralizations that leave no acids or bases behind. To understand the controlling of pH of solutions, buffers are discussed in the laboratory and in the chemistry of the bloodstream. Part of Chemistry: Challenges and Solutions Series.
(Source: DCMP)
-
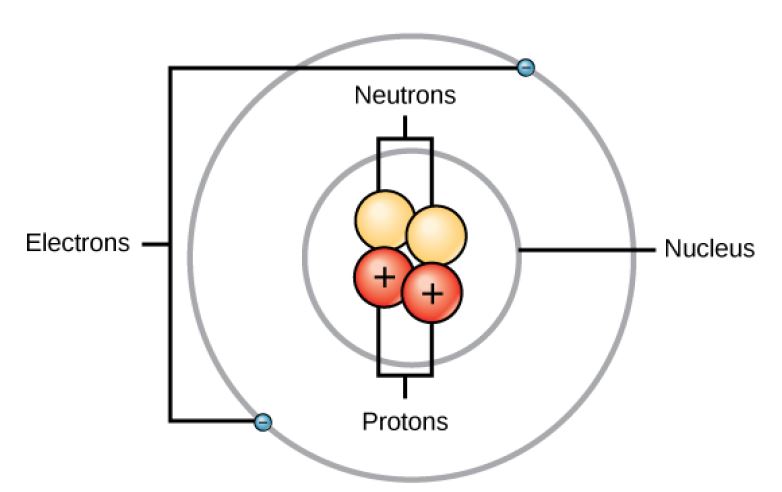
Atom Diagram
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.2 (OpenStax, Biology 2e) caption: Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
(Source: OpenStax)
-
-
Atom: Clash Of Titans
-
 Video
Video

As scientists delved deep into the atom, into the very heart of matter, they unraveled nature's most shocking secrets. They had to abandon everything they believed in and create a whole new science, which today underpins the whole of physics, chemistry, biology, and maybe even life itself. Tells a story of great geniuses, like Albert Einstein and Werner Heisenberg who were driven by their thirst for knowledge and glory. It's a story of false starts and conflicts, ambition, and revelation, a story which leads us through some of the most exciting and exhilarating ideas ever conceived of by the human race.
(Source: DCMP)
-
-
Atoms
-
 Video
Video

What is an atom? It is the smallest particle of an element, and everything is made up of atoms. They consist of three basic particles: protons, electrons, and neutrons. The scientific community has experienced significant breakthroughs which have contributed to the understanding of atoms. Other topics covered include atomic number, atomic mass, Bohr model, electron cloud, and isotope.
(Source: DCMP)
-
-
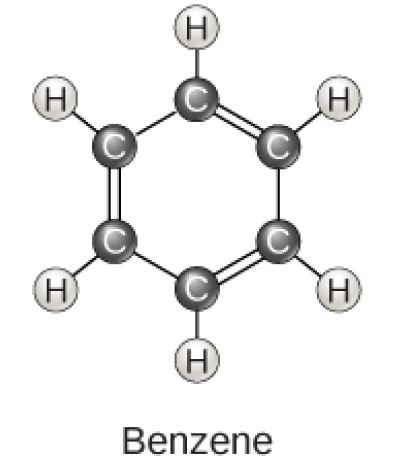
Benzene Molecular Diagram
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.23 (OpenStax, Biology 2e) caption: Carbon can form five- and six-membered rings. Single or double bonds may connect the carbons in the ring, and nitrogen may be substituted for carbon.
(Source: OpenStax)
-
-
Bohr Atomic Model
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.6 (OpenStax, Biology 2e) caption: In 1913, Niels Bohrs developed the Bohr model in which electrons exist within principal shells. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable, and the electron quickly decays back to the ground state. In the process, it releases a photon of light.
(Source: OpenStax)
-
-
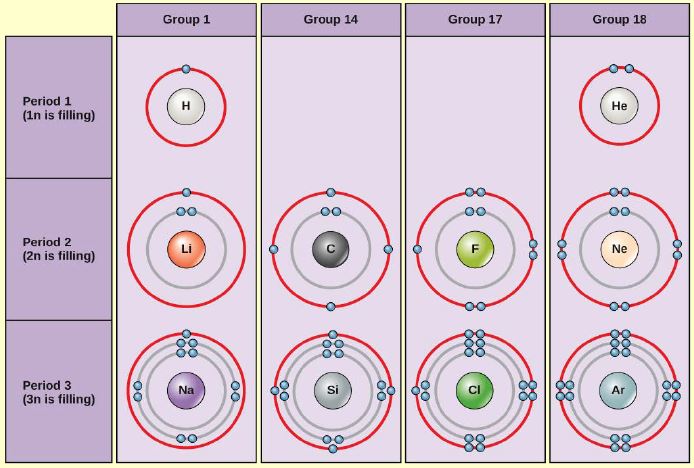
Bohr Diagrams
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.7 (OpenStax, Biology 2e) caption: Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration.
(Source: OpenStax)
-
-
Bonding
-
 Video
Video

Part of the "Chemistry in Action" series. Describes and illustrates the process of chemical bonding through live action footage and animations. Provides an overview of the role atomic structure plays in the process of bonding. Provides examples of how chemical bonding, including ionic bonds, covalent bonds, and metallic bonds, affects the characteristics of matter. Introduces the following terminology: element, atomic structure, energy level, valence electrons, ionic bonds, crystal lattice, covalent bond, and metallic bond.
(Source: DCMP)
-
-

Experiments show the production and properties of carbon dioxide and its use as a fire extinguisher. The second part demonstrates the effect of surface area, temperature, and concentration on chemical reaction rates.
(Source: DCMP)
-
Chemical Changes
-
 Video
Video

From enjoying the warmth of a fire to baking a cake, people benefit from chemical changes every day. This program illustrates and explains numerous examples of chemical changes. It also takes a look at some of the common characteristics of reactions that occur chemically. Concepts and terminology explored include: physical change, chemical change, reaction, color change, gas, and heat.
(Source: DCMP)
-
-

Very little in the physical world around us occurs without chemical reactions being involved. Takes an in-depth look at five common products that are in use all around us. Also, explores the chemistry behind their manufacture and/or use, including chemical equations. The products are soap, polystyrene, aluminum, paint, and car batteries.
(Source: DCMP)
-
Chemistry (Program 2)
-
 Video
Video

The formation of molecular bonds is an essential part of keeping matter together. The sharing of charges between atoms helps them become more stable. Other topics covered include sharing electrons, bonding tendencies, isomers, VSEPR theory, and molecular geometry. Part of the "Chemistry" series.
(Source: DCMP)
-
-
Chemistry (Program 3)
-
 Video
Video

The state of matter is the form taken by matter at a given temperature and pressure. A phase of matter is uniform with respect to its physical and chemical properties. Matter undergoes phase transitions to change from one phase to another. Part of the "Chemistry" series.
(Source: DCMP)
-
-
Chemistry (Program 4)
-
 Video
Video

A solution is a homogeneous mixture of two or more substances. It contains a solute and and solvent. Solubility is the maximum quantity of a solute that can be dissolved in a given amount of solvent at a specified temperature. Other topics covered include concentration, Raoult's law, colligative properties, and non-ideal behavior. Part of the "Chemistry" series.
(Source: DCMP)
-
-
Chemistry (Program 5)
-
 Video
Video
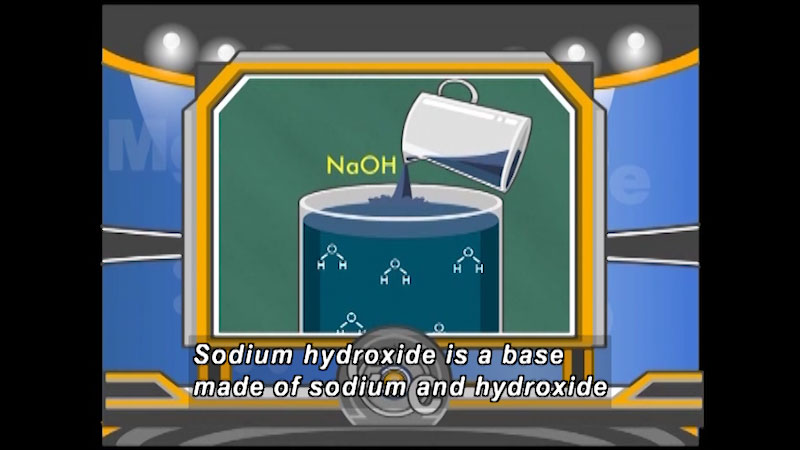
Solutions are classified as acidic or basic based on their hydrogen ion concentration relative to pure water. An acidic solution has a higher concentration of hydrogen ions, and a basic solution has a lower concentration of hydrogen ions. Other topics covered include chemical reaction basics, properties of acids and based, acid-base reactions, and other mixtures. Part of the "Chemistry" series.
(Source: DCMP)
-
-
Chemistry (Program 6)
-
 Video
Video

A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Topics covered include precipitation reactions, oxidation-reduction reactions, kinetics, equilibrium, and nuclear reactions. Part of the "Chemistry" series.
(Source: DCMP)
-
-
Chemistry (Program 7)
-
 Video
Video
Using a balanced chemical equation to calculate amounts of reactants and products is called stoichiometry. It is a super technical-sounding word that simply means using ratios from the balanced equation. Topics covered in this program include the metric system, balancing equations, molar conversions, mass percent, empirical formulas, and limiting reactants. Part of the "Chemistry" series.
(Source: DCMP)
-
-
Chemistry (Program 8)
-
 Video
Video

Thermodynamics deals broadly with the conservation and conversion of various forms of energy. It also describes the relationships between energy and the changes in properties of matter. Other topics covered include Hess's law, the laws of thermodynamics, and the Gibbs free energy. Part of the "Chemistry" series.
(Source: DCMP)
-
-
Compounds In Chemistry
-
 Video
Video
Part of the "Chemistry in Action" series. Demonstrates how chemical compounds are placed into groups so that they may be studied easier. Explores acids and bases, emphasizing their nature and common everyday uses. Discusses carbon compounds, and introduces the following terminology: acid, base, pH, salt, carbon, organic, and hydrocarbon.
(Source: DCMP)
-
-
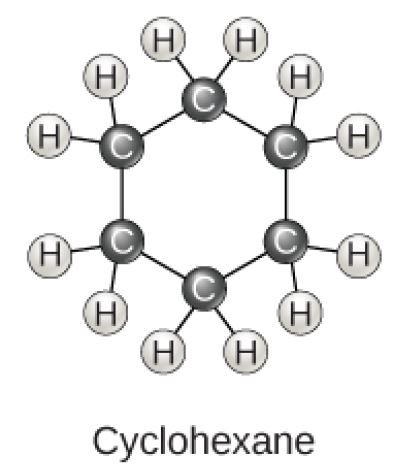
Cyclohexane Molecular Diagram
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.23 (OpenStax, Biology 2e) caption: Carbon can form five- and six-membered rings. Single or double bonds may connect the carbons in the ring, and nitrogen may be substituted for carbon.
(Source: OpenStax)
-
-
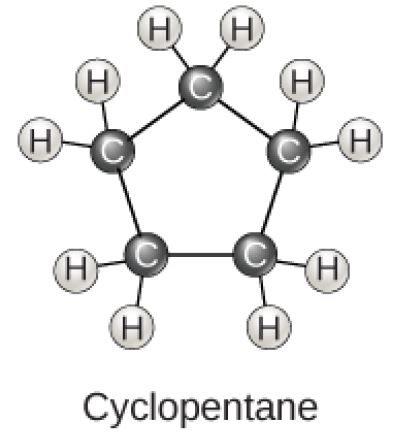
Cyclopentane Molecular Diagram
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.23 (OpenStax, Biology 2e) caption: Carbon can form five- and six-membered rings. Single or double bonds may connect the carbons in the ring, and nitrogen may be substituted for carbon.
(Source: OpenStax)
-
-

With today's high fuel costs, it is time to start looking beyond petroleum and into renewable resources to power vehicles. Ethanol is a clean-burning fuel derived from plants, primarily corn. It is combined with gasoline to produce a cleaner fuel that doesn't emit as many greenhouse gases as pure gasoline. Pure ethanol has not yet been approved to fuel vehicles, but it is the fuel of choice for racecars. By mixing gasoline with ethanol, fuel supply can be extended. Explores the production of ethanol and highlights the importance it holds in the "green power" movement.
(Source: DCMP)
-

This video explains the vital role elements and compounds play in making up matter. Everyday examples of different types of mixtures help students realize the important role they play in daily life. Other topics covered include elements, compounds, homogeneous and heterogeneous mixtures, colloid, suspension, solution, solvent, and solute.
(Source: DCMP)
-
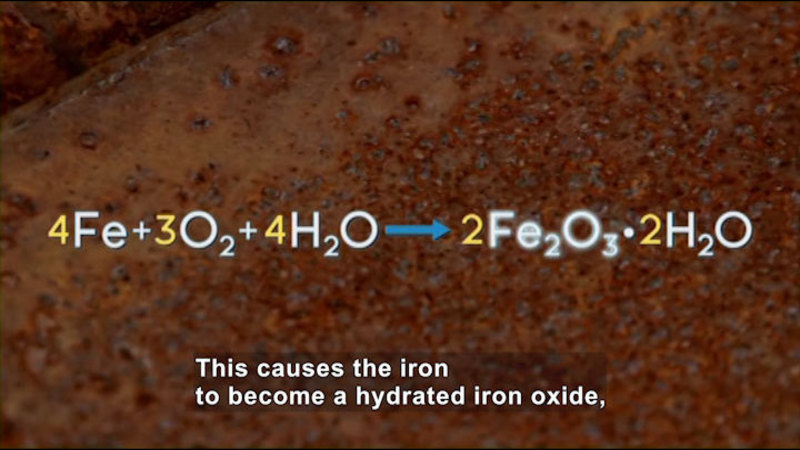
Some chemical reactions happen spontaneously, like metal rusting. Other reactions are non-spontaneous and need to absorb energy in order to occur. Using the Second Law of Thermodynamics, the principle of entropy, and the calculation of Gibbs free energy, scientists can predict which reactions will occur and vary the conditions to make more of the desired products. In equilibrium reactions, both products and reactants are always present. Equilibrium reactions in the human body are essential for life and can be exploited in chemical manufacturing as well. Part of the series Chemistry: Challenges And Solutions.
(Source: DCMP)
-

Tests the heat zones of a Bunsen burner and shows some elements' coloration when placed in the flame. Notes that chemistry is everywhere, in both natural and man-made components.
(Source: DCMP)
-
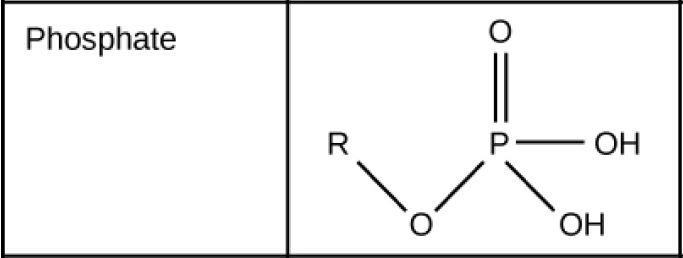
Function Group: Phosphate
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.27 (OpenStax, Biology 2e) caption: These functional groups are in many different biological molecules. R, also known as R-group, is an abbreviation for any group in which a carbon or hydrogen atom is attached to the rest of the molecule.
(Source: OpenStax)
-
-
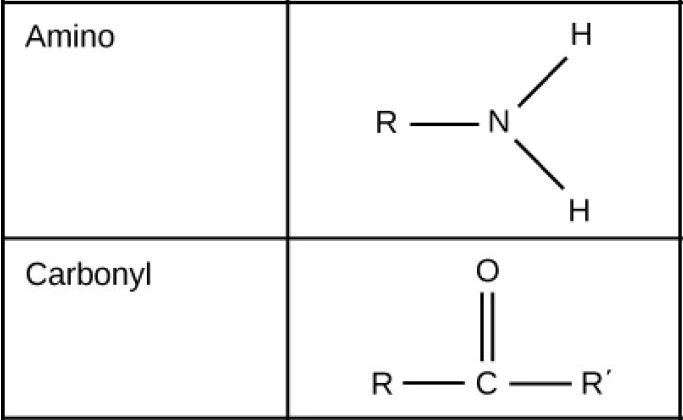
Function Groups: Amino and Carbonyl
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.27 (OpenStax, Biology 2e) caption: These functional groups are in many different biological molecules. R, also known as R-group, is an abbreviation for any group in which a carbon or hydrogen atom is attached to the rest of the molecule.
(Source: OpenStax)
-
-
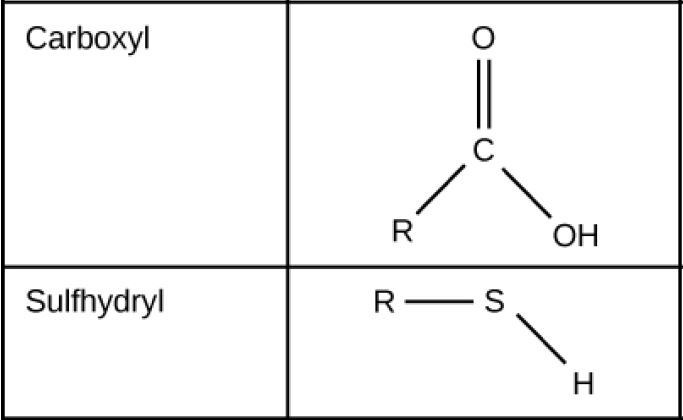
Function Groups: Carboxyl and Sulfhydryl
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.27 (OpenStax, Biology 2e) caption: These functional groups are in many different biological molecules. R, also known as R-group, is an abbreviation for any group in which a carbon or hydrogen atom is attached to the rest of the molecule.
(Source: OpenStax)
-
-
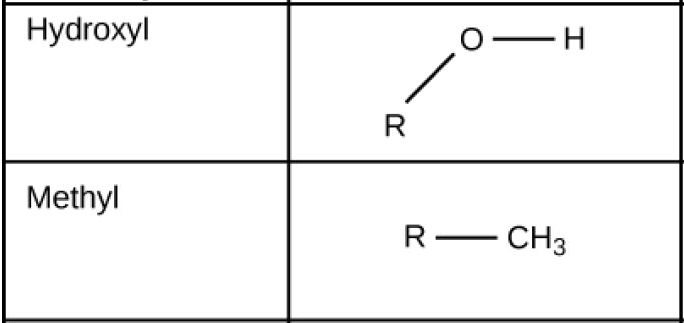
Function Groups: Hydroxyl and Methyl
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.27 (OpenStax, Biology 2e) caption: These functional groups are in many different biological molecules. R, also known as R-group, is an abbreviation for any group in which a carbon or hydrogen atom is attached to the rest of the molecule.
(Source: OpenStax)
-
-
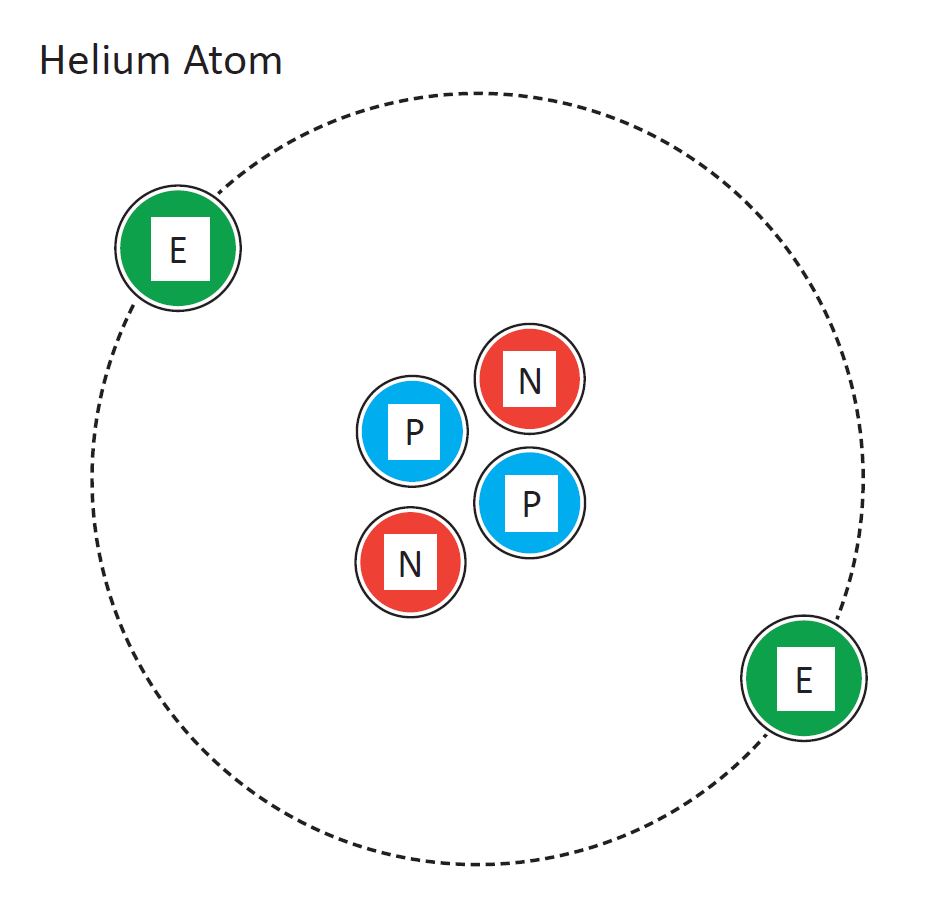
Helium
-
 Image
Image
-
 Text Document
Text Document
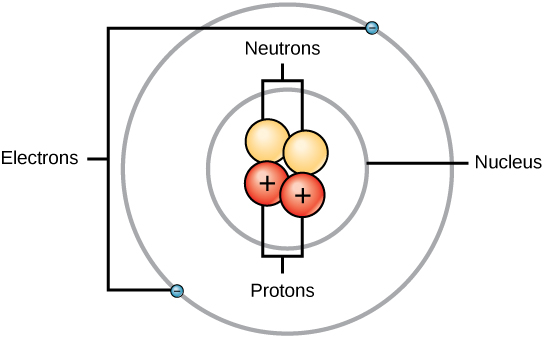
Remixed from Customizable Atom Delux by roman_hegglin. Helium is a chemical element with symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas, the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the elements.
(Source: OpenStax)
-
-
Helium Atomic Diagram
-
 Image
Image
-
 Text Document
Text Document
-
 PDF
PDF
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
Diagram of a helium atom. Design modalities for the image include braille with and without labels, print with and without labels in greyscale, color, and texture.
(Source: Benetech)
-
-
Hydrocarbons
-
 Video
Video

Lab experiments demonstrate a variety of ways to detect carbon and hydrogen in organic substances. Burns hexane, benzene, cyclohexane, and naphthalene; shows properties of a propane-butane mixture.
(Source: DCMP)
-
-
Hydrogen
-
 Video
Video

Demonstrates the production and collection of hydrogen in a chemistry lab, and testing of its purity. Also shows and explains other chemical reactions related to this element.
(Source: DCMP)
-
-
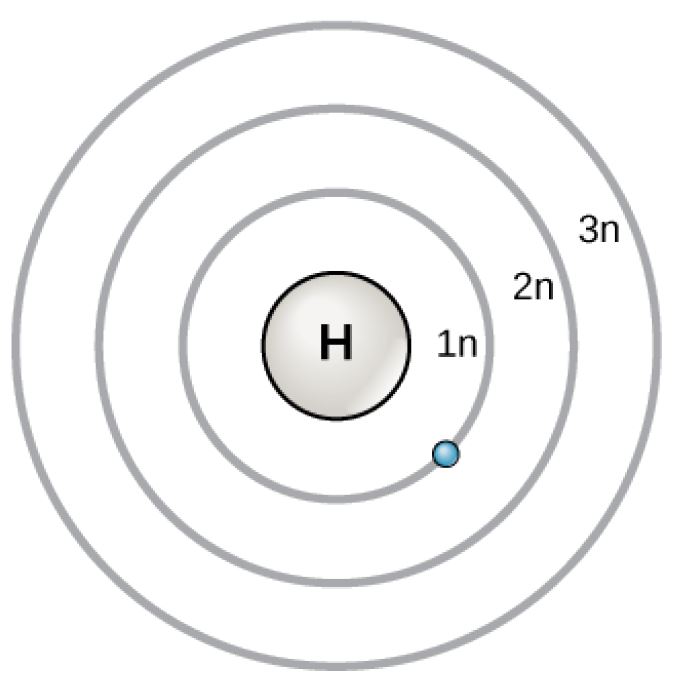
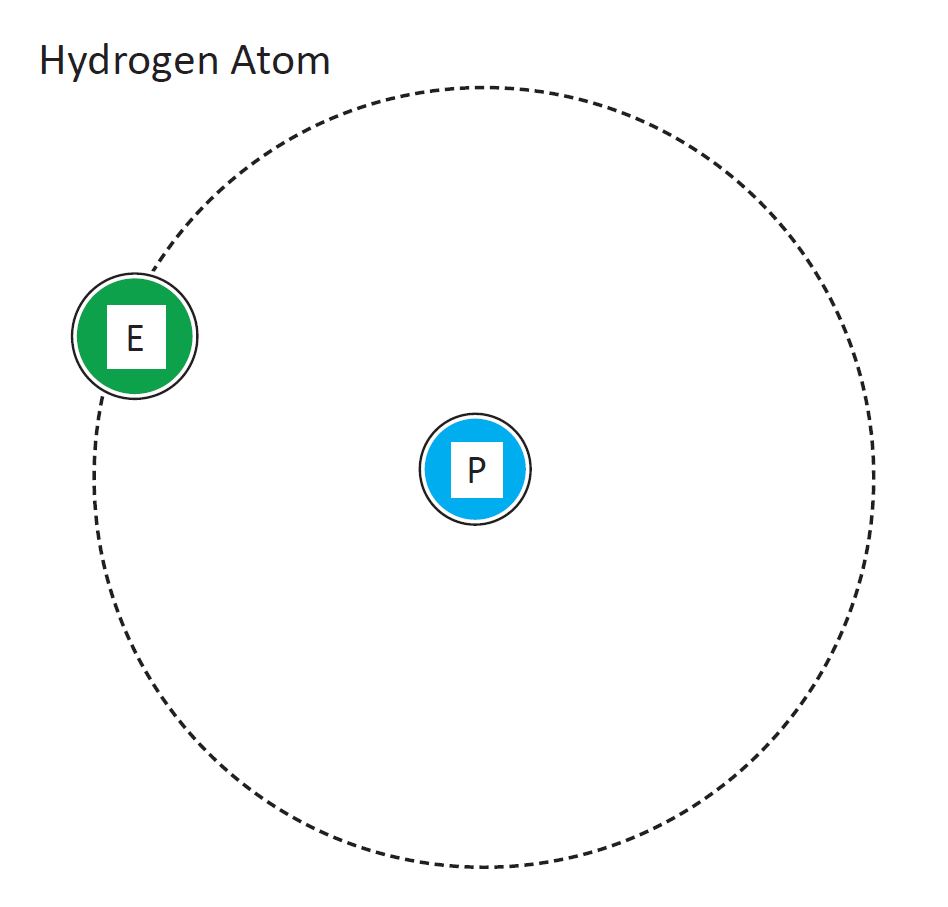
Hydrogen Atomic Diagram
-
 Image
Image
-
 Text Document
Text Document
-
 PDF
PDF
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
Diagram of a hydrogen atom. Design modalities for the image include braille with and without labels, print with and without labels in greyscale, color, and texture.
(Source: Benetech)
-
-

Carbon is the basis of all organic molecules. It is also one of the most abundant elements in the universe. This video segment illustrates the special characteristics of carbon that make it an essential ingredient for life.
(Source: DCMP)
-

From cooking food to enjoying the warmth of a fire, chemical reactions happen every day. Students learn the major characteristics and types of chemical reactions. Additional concepts and terminology discussed include: reactants, products, physical and chemical changes, chemical equation, reaction rate, and indicators of chemical reactions.
(Source: DCMP)
-

Students learn how to differentiate mixtures, solutions, elements, and compounds. Additional concepts and terminology discussed include: element, pure substance, properties, atoms, molecule, compounds, types of mixtures, suspension, colloid, and alloy.
(Source: DCMP)
-
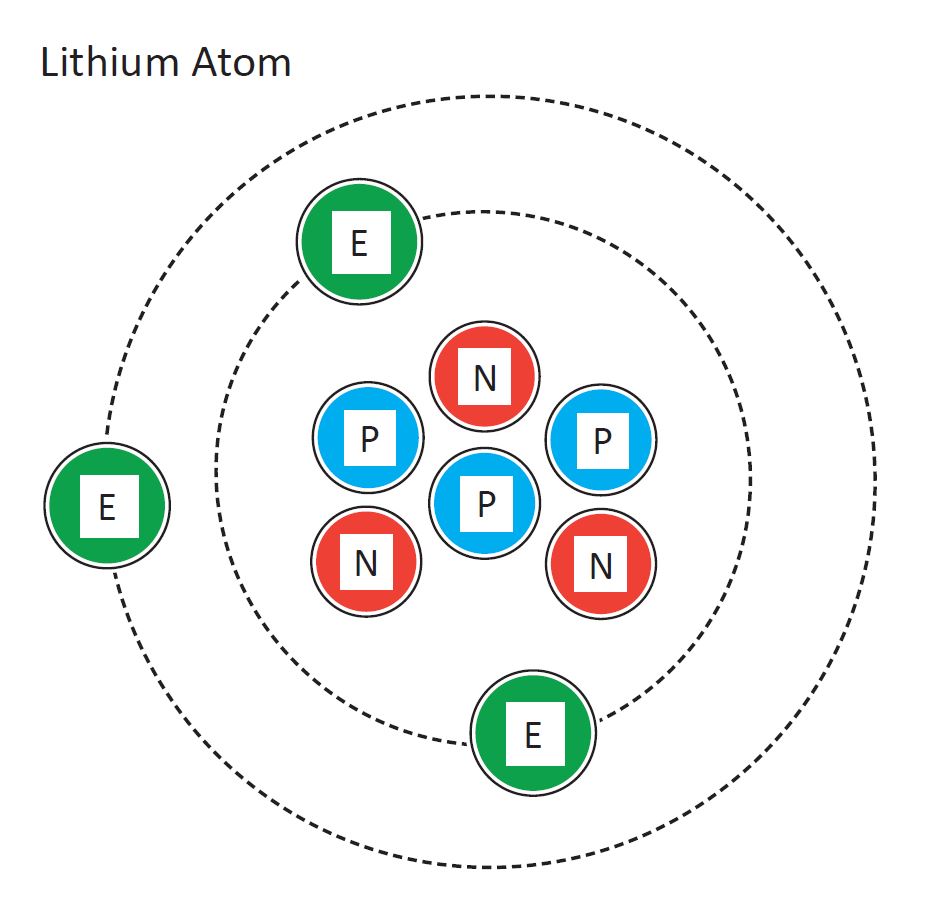
Lithium Atomic Diagram
-
 Image
Image
-
 Text Document
Text Document
-
 PDF
PDF
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
Diagram of a lithium atom. Design modalities for the image include braille with and without labels, print with and without labels in greyscale, color, and texture.
(Source: Benetech)
-
-
Metals I
-
 Video
Video

Lab experiments show how magnesium, zinc, iron, and copper react with hydrochloric and nitric acids. Also shows the displacement of two metals from salts.
(Source: DCMP)
-
-
Metals 2
-
 Video
Video

Shows the combustion of magnesium, a thermite reaction to form iron, and the chemical reactions of sodium and potassium with water.
(Source: DCMP)
-
-
Molarity
-
 Simulation
Simulation
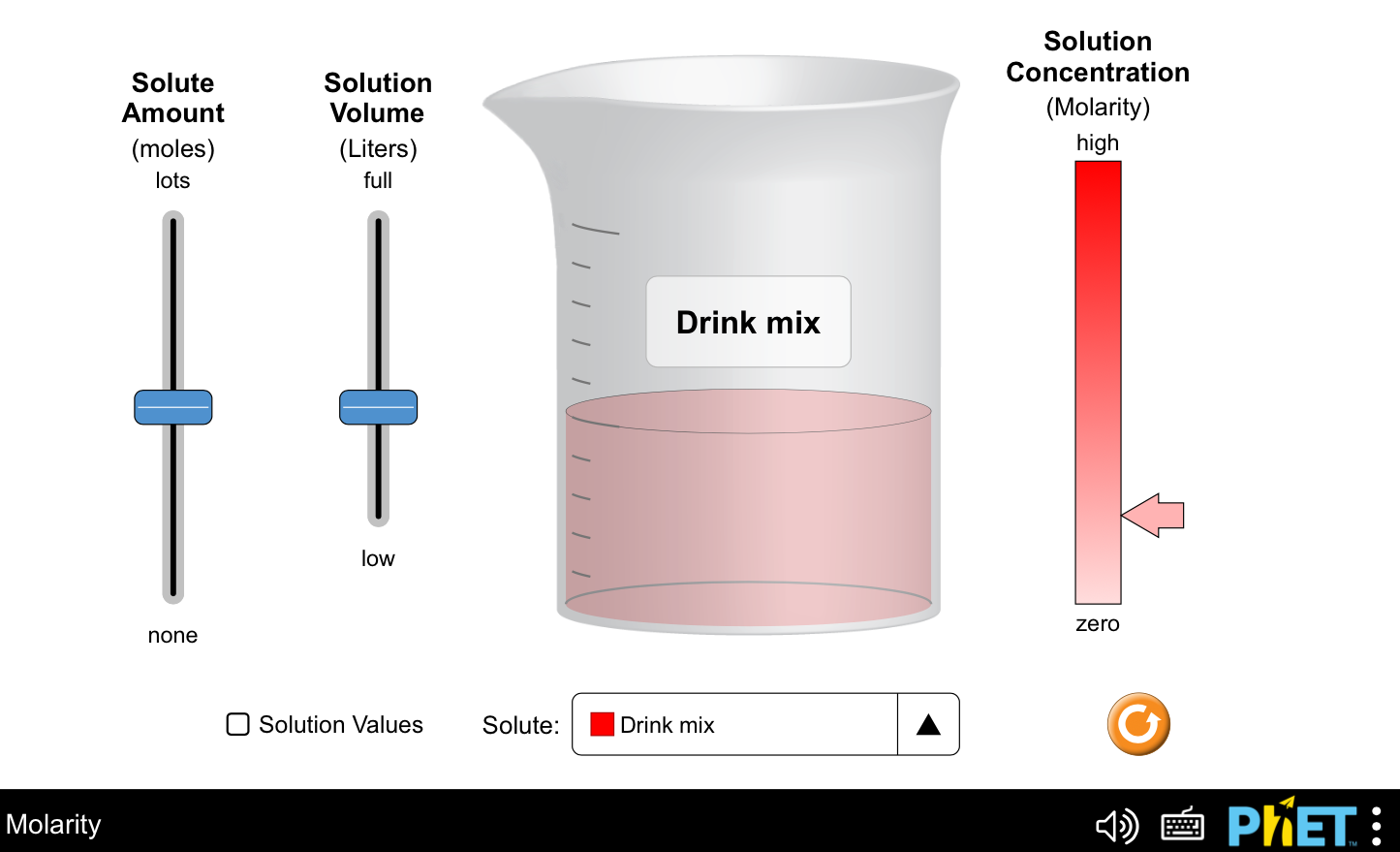
What is concentration? Explore the relationships between moles, liters, and molarity by adjusting the solute amount, solution volume, and changing solutes to compare different chemical compounds in water.
(Source: PhET Interactive Simulations)
-
-
Nonmetals
-
 Video
Video

Experiments feature: (1) halogens as oxidizing agents, (2) bromine reacting with potassium, (3) detecting bromine in compounds, (4) combustion of sulphur and the formation of sulfuric acid, (5) properties of phosphorus, and (6) spontaneous ignition of white phosphorus.
(Source: DCMP)
-
-
Organic Acids
-
 Video
Video

Organic acids, found in common foods, appear as citric acid (lemons), acetic acid (vinegar), and malic acid (apples). Also shows esterification and the formation of an ester.
(Source: DCMP)
-
-
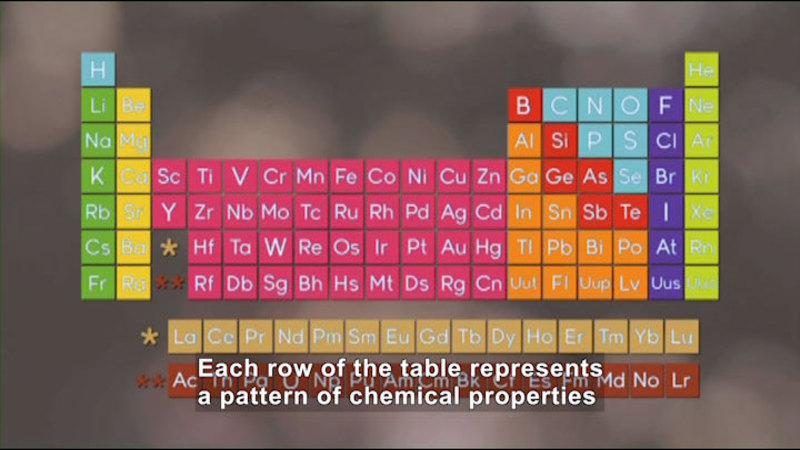
As scientists discovered more and more chemical elements, they began developing systems to organize the elements by their chemical properties, leading to the modern periodic table. Through its organization, the periodic table makes clear the underlying chemical and physical trends among the elements. The periodic table is being continually updated even today as scientists strive to create new elements in laboratories. Part of the series Chemistry: Challenges And Solutions.
(Source: DCMP)
-
Oxygen
-
 Video
Video

Lab experiments test for oxygen and show its reaction to iron and carbon.
(Source: DCMP)
-
-
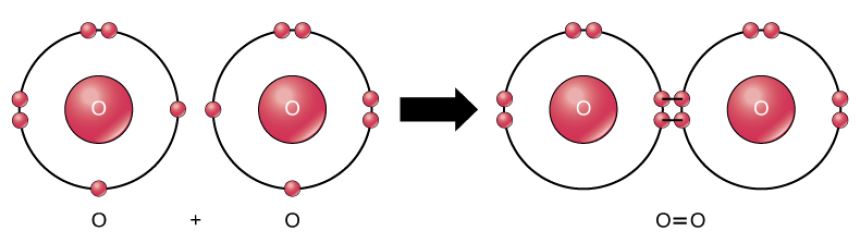
Oxygen Atoms
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.10 (OpenStax, Biology 2e) caption: A double bond joins the oxygen atoms in an O2 molecule.
(Source: OpenStax)
-
-
Everything is made of millions of tiny particles. Animations illustrate how matter consists of different types of particles that are responsible for varying characteristics. Concepts and terminology include atom, element, chemical, and compound.
(Source: DCMP)
-
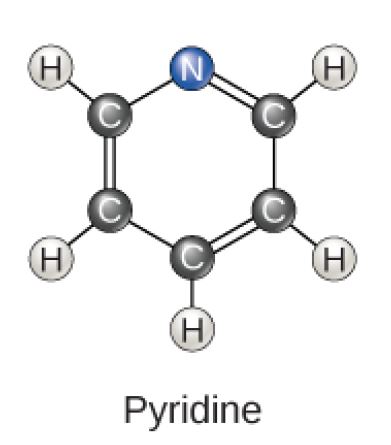
Pyridine Molecular Diagram
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.23 (OpenStax, Biology 2e) caption: Carbon can form five- and six-membered rings. Single or double bonds may connect the carbons in the ring, and nitrogen may be substituted for carbon.
(Source: OpenStax)
-
-
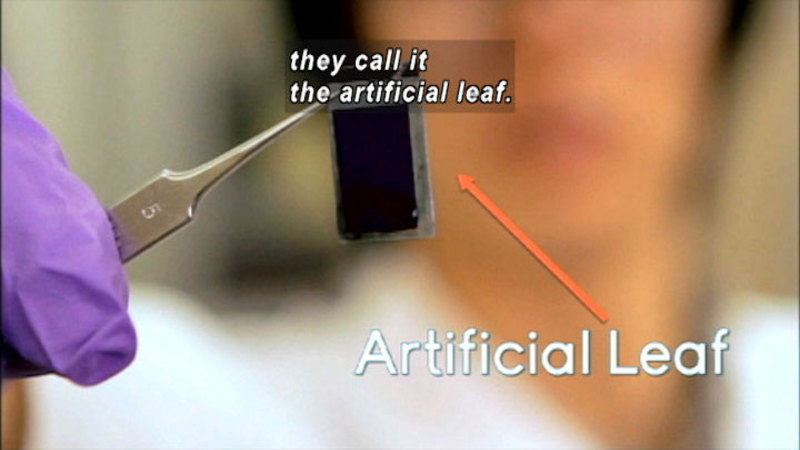
To manipulate chemical reactions on a large scale, scientists use stoichiometry to quantify those reactions. The use of stoichiometry ensures there are the right amount of reactants and products. Without it, reactions can be incomplete, with expensive materials wasted and harmful byproducts created. Using stoichiometry, scientists are creating chemicals that take the place of petroleum in fabricating sustainable materials. At a different lab, scientists are mimicking the process of photosynthesis to convert the sun’s energy into storable chemical energy. Part of Chemistry: Challenges and Solutions Series.
(Source: DCMP)
-
Radioactivity
-
 Video
Video
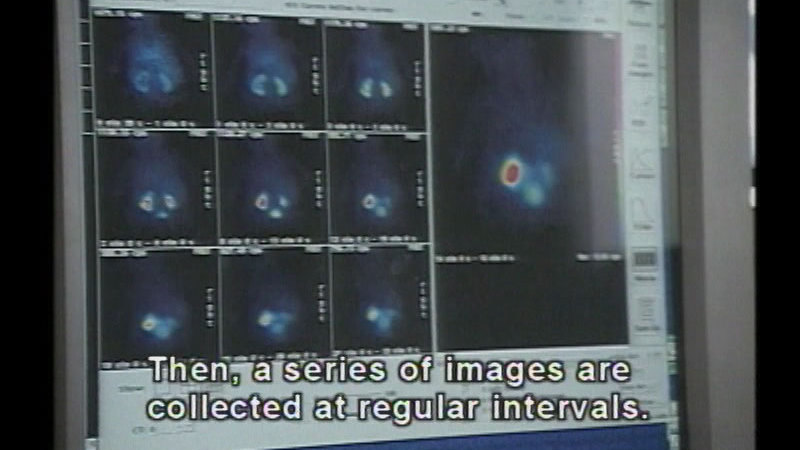
Radioactivity is all around us and comes from a variety of sources. There are three types of radiation, and experiments show the differences in the penetrating power of each one. A major use of radioactivity is in nuclear medicine. Discusses the half-life of radiation and how to calculate it.
(Source: DCMP)
-
-
Reactions
-
 Video
Video

Part of the "Chemistry in Action" series. Chemicals interacting with each other are one of the most fascinating topics in chemistry. Fireworks, burning flares, and rusting all illustrate chemical reactions. Describes the different types of reactions as well as the process of balancing chemical equations. Introduces the following terminology: chemical equations, Law of Conservation of Mass, decomposition and synthesis reactions, replacement reactions, and reaction rates.
(Source: DCMP)
-
-

Presents three key concepts about chemical reactions and energy changes: exothermic and endothermic reactions, reaction rates involving temperature and concentration, and catalysts. Each concept is illustrated with a variety of experiments and computer animation to illuminate what is happening both visibly and at the molecular level.
(Source: DCMP)
-

Anything that takes up space or has mass is matter. Under certain conditions matter can be a solid, liquid, gas or plasma. Different states of matter can be combined in suspensions, and solutions and mixtures can be taken apart. Exploring the physical and chemical properties of matter provides insight into nature and a glimpse at how scientists and engineers use this knowledge to shape our world.
(Source: DCMP)
-
What are the differences between solids, liquids, and gases? In this program, students will investigate real-life examples of the various phases of matter. Colorful animations illustrate how these states differ based on the movement of particles. Other topics covered include plasma, crystalline and amorphous solids, viscosity, freezing, vaporization, evaporation, and condensation.
(Source: DCMP)
-

Chemistry is the science of interacting particles and the various states of matter. Developing a better understanding of the atomic model through experiments with gases, scientists discovered the Ideal Gas Law, developed phase diagrams, and learned about the properties of supercritical fluids. Today's chemists are exploring new ways to control the interactions of atoms, with the goal of making better hydrogen-powered cars and new technologies for the long-term, underground storage of carbon dioxide to reduce greenhouse warming. Part of the series Chemistry: Challenges And Solutions.
(Source: DCMP)
-

Sherlock Olmos decides to dig deeper into dark corner of this great house to investigate the suspicious behavior of some chemical elements. The exchange of electrons and the characteristics of hydrogen, fluorine gas, and the noble gases are the primary subjects of his investigation. Part of Chemistry: Solved by Sherlock Olmos Series.
(Source: DCMP)
-

Sherlock Olmos uses his famous detective skills to solve his mysterious case of how the periodic table is structured. With a touch of humor, he investigates electrons, valences, and the physical and chemical properties of some of the elements. Part of Chemistry: Solved by Sherlock Olmos Series.
(Source: DCMP)
-
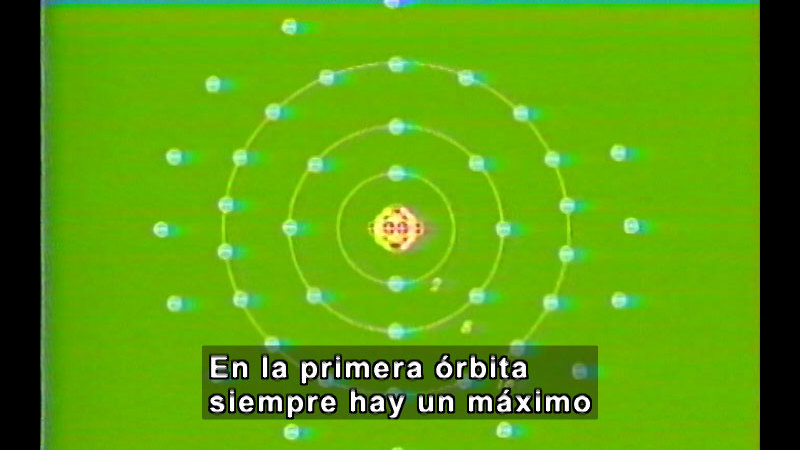
Sherlock Olmos uses his famous detective skills to solve his mysterious case of how the periodic table is structured. With a touch of humor, he investigates electrons, valences, and the physical and chemical properties of some of the elements. Part of Chemistry: Solved by Sherlock Olmos Series.
(Source: DCMP)
-

The study of thermodynamics can lead to predicting how chemical reactions will proceed or how much energy is required or released during the reactions. To better understand chemical reactions, a new thermodynamic value called “enthalpy” is introduced. Students will examine the practical applications of bond enthalpies, calorimetry, and other measurements of the energy in chemical reactions. They will also see how the understanding of thermodynamics and enthalpy is helping scientists optimize the use of crop waste for biofuels and build more efficient automobile engines. Part of Chemistry: Challenges and Solutions Series.
(Source: DCMP)
-
Since the beginning of time, humans have used chemical reactions without understanding them. For example, ancient Greek artisans were able to smelt metal, dye fabrics, and make glass. The attempt to transform simple metal into gold and silver was known as alchemy (the forerunner of chemistry as we know it today). By mixing elements, alchemists created chemical reactions which produced new compounds. While alchemists were never able to transform anything into gold or silver, their trails helped shape the science of chemistry. Part of Chemistry: Solved by Sherlock Olmos Series.
(Source: DCMP)
-
The Periodic Table
-
 Video
Video

Part of the "Chemistry in Action" series. Numerous real-life examples of elements are exemplified in the table. Animations and graphics illustrate concepts not easily achieved through other instructional strategies. Specific patterns in the periodic table are highlighted. Introduces the following terminology: atomic structure, atomic number, atomic mass, periods, and families.
(Source: DCMP)
-
-

Science lab experiments demonstrate what happens visibly and at a molecular level with the Group 1 highly reactive metals and the Group 17 highly reactive halogens from the periodic table. Experiments also illustrate the range of reactivity among metals. Video has three 5-minute segments for convenience.
(Source: DCMP)
-
Molecules form when individual atoms create bonds by sharing electrons. Understanding how atoms combine to make molecules allows scientists to predict many of the physical and chemical properties of substances. Since the outermost eight electrons are key to forming compounds, this unit shows how the Octet Rule provides a basis for predicting how atoms may gain, lose, or share electrons to fill the slots in their outer shells. A fundamental understanding of how electrons form bonds leads to the three-dimensional shapes of molecules and has implications in all aspects of chemistry. Part of the series Chemistry: Challenges And Solutions.
(Source: DCMP)
-
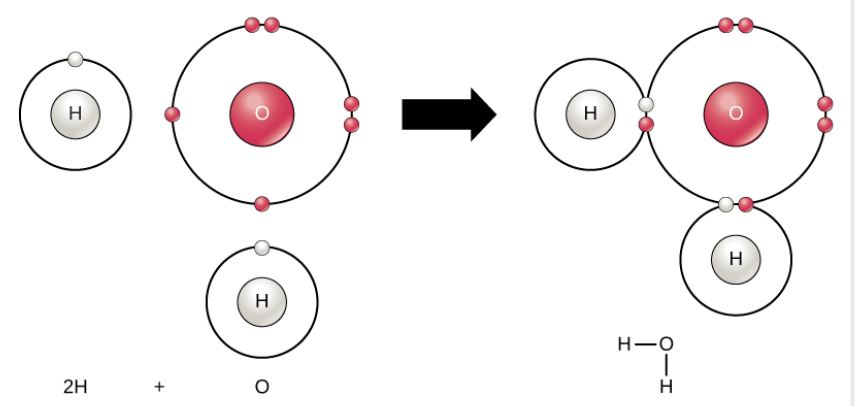
Water Molecule
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
Figure 2.9 (OpenStax, Biology 2e) caption: Two or more atoms may bond with each other to form a molecule. When two hydrogens and an oxygen share electrons via covalent bonds it forms a water molecule.
(Source: OpenStax)
-
-
What Are Atoms Made Of?
-
 Video
Video

It's called a theory, but if we have never seen an atom, how did anyone ever come up with an idea that is so central to science. Shows how all the pieces of the puzzle have come together at the same time, explaining the structure of the atom and the periodic table.
(Source: DCMP)
-
-

Solutions are uniform mixtures of molecules in which any of the phases of matter can be dissolved in another phase. Whether solids, liquids, or gases, solution chemistry is important because most chemical reactions, whether in the laboratory or in nature, take place in solutions. In particular, solutions with water as the solvent are the core of all biology. Extending the particle model of matter to solutions enables chemists to predict what will happen to a deep-sea diver who breathes different mixtures of gases or to the life forms in the ocean as carbon dioxide levels rise in the atmosphere. Part of Chemistry: Challenges and Solutions Series.
(Source: DCMP)
-
Wild Chronicles: Carbon
-
 Video
Video

The scientific explanation of global warming rests in the understand of the element carbon. Carbon is the central element of life, and its atomic structure enables it to hold onto other elements. This characteristic provides the relationship between carbon and global warming. Segment of video from Wild Chronicles Series.
(Source: DCMP)
-