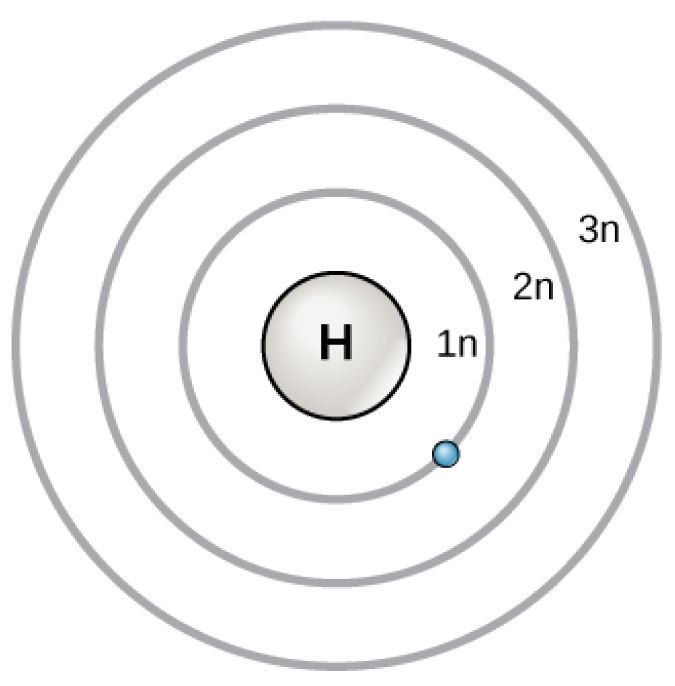
Bohr Atomic Model
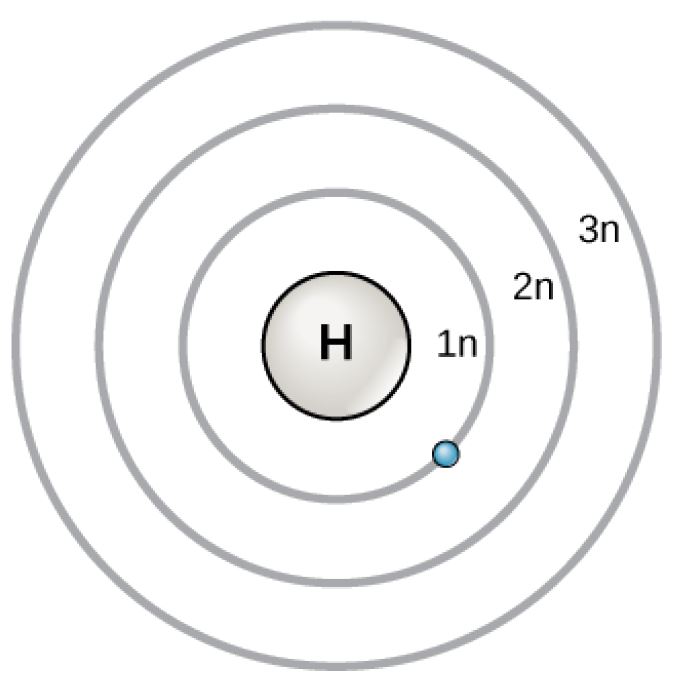
Figure 2.6 (OpenStax, Biology 2e) caption: In 1913, Niels Bohrs developed the Bohr model in which electrons exist within principal shells. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable, and the electron quickly decays back to the ground state. In the process, it releases a photon of light.
(Source: OpenStax)
Metadata
- Subject:
- Physical Sciences - Science
Files 4
Download all files (4)-
Bohr Atomic Model Original Image
Bohr Atomic Model Original Image
- Type:
- Image
- Format:
- Image - JPG
- Accommodations:
- Visual
- Languages:
- English
- License:
- CC:BY 4.0
-
Bohr Atomic Model Adobe Illustrator

Bohr Atomic Model Adobe Illustrator
- Type:
- 2.5D Tactile Graphic
- Format:
- Tactile - AI
- Accommodations:
- Tactile Graphic with Braille - Visual
- Languages:
- English
- License:
- CC:BY-NC
- Author:
- Nicole Johnson
-
Bohr Atomic Model PDF

Bohr Atomic Model Tactile Graphic
- Type:
- 2.5D Tactile Graphic
- Format:
- Other - PDF
- Accommodations:
- Tactile Graphic with Braille - Visual
- Languages:
- English
- License:
- CC:BY-NC
- Author:
- Nicole Johnson
-
Bohr Atomic Model Description

Bohr Atomic Model Description
- Type:
- Text Document
- Format:
- Other - TXT
- Accommodations:
- Image Description - Visual
- Languages:
- English
- License:
- CC:BY 4.0
Collections 1
This resource is part of one or more collections.
-
Chemistry -
 Video
Video
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
-
 Simulation
Simulation
A collection of Chemistry related resources
A collection containing 67 resources, curated by Benetech
-