The Structure Of Molecules: Lewis Structures And Molecular Geometries
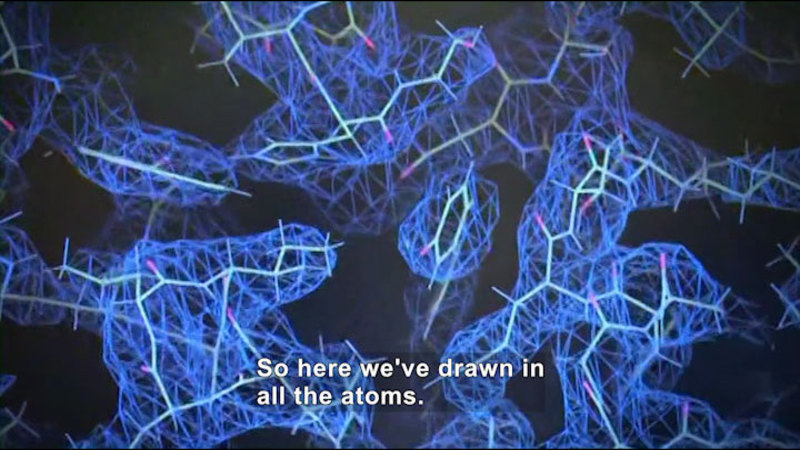
Molecules form when individual atoms create bonds by sharing electrons. Understanding how atoms combine to make molecules allows scientists to predict many of the physical and chemical properties of substances. Since the outermost eight electrons are key to forming compounds, this unit shows how the Octet Rule provides a basis for predicting how atoms may gain, lose, or share electrons to fill the slots in their outer shells. A fundamental understanding of how electrons form bonds leads to the three-dimensional shapes of molecules and has implications in all aspects of chemistry. Part of the series Chemistry: Challenges And Solutions.
(Source: DCMP)
Metadata
- Subject:
- Physical Sciences - Science
- Keywords:
- atoms, chemistry, electrons, molecules, science methods
Files 1
-
The Structure Of Molecules: Lewis Structures And Molecular Geometries

- Type:
- Video
- Format:
- Streaming
- Accommodations:
- English Audio Descriptions - Visual, English Captions - Auditory
- Languages:
- English
- License:
- DCMP Membership
- Author:
- Annenberg
- Length:
- 29 minutes
Collections 1
This resource is part of one or more collections.
-
Chemistry -
 Video
Video
-
 Image
Image
-
 2.5D Tactile Graphic
2.5D Tactile Graphic
-
 PDF
PDF
-
 Text Document
Text Document
-
 Simulation
Simulation
A collection of Chemistry related resources
A collection containing 67 resources, curated by Benetech
-
