Ionic Compound
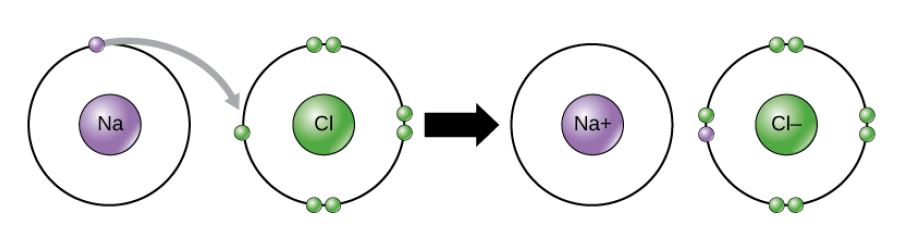
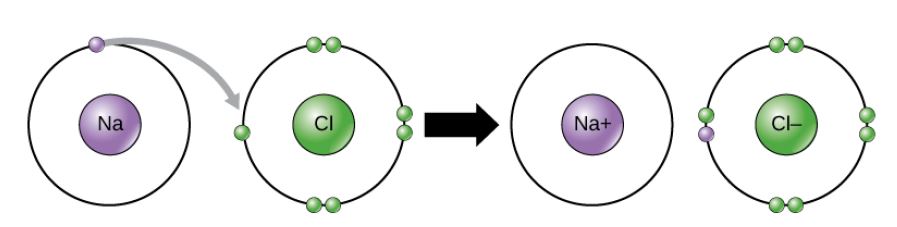
Figure 2.11 (OpenStax, Biology 2e) caption: In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet.
(Source: OpenStax)
Metadata
- Subject:
- Physical Sciences - Science
Files 4
Download all files (4)-
Ionic Compound Original Figure
Ionic Compound Original Figure
- Type:
- Image
- Format:
- Image - JPG
- Accommodations:
- Visual
- Languages:
- English
- License:
- CC:BY 4.0
-
Ionic Compound Figure Illustrator

Ionic Compound Figure Illustrator
- Type:
- 2.5D Tactile Graphic
- Format:
- Tactile - AI
- Accommodations:
- Tactile Graphic with Braille - Visual
- Languages:
- English
- License:
- CC:BY-NC
- Author:
- Nicole Johnson
-
Ionic Compound Figure PDF

Ionic Compound Figure PDF
- Type:
- 2.5D Tactile Graphic
- Format:
- Other - PDF
- Accommodations:
- Tactile Graphic with Braille - Visual
- Languages:
- English
- License:
- CC:BY-NC
- Author:
- Nicole Johnson
-
Ionic Compound Figure Description

Ionic Compound Figure Description
- Type:
- Text Document
- Format:
- Other - TXT
- Accommodations:
- Image Description - Visual
- Languages:
- English
- License:
- CC:BY 4.0