Kinetics and Nuclear Chemistry: Rates Of Reaction
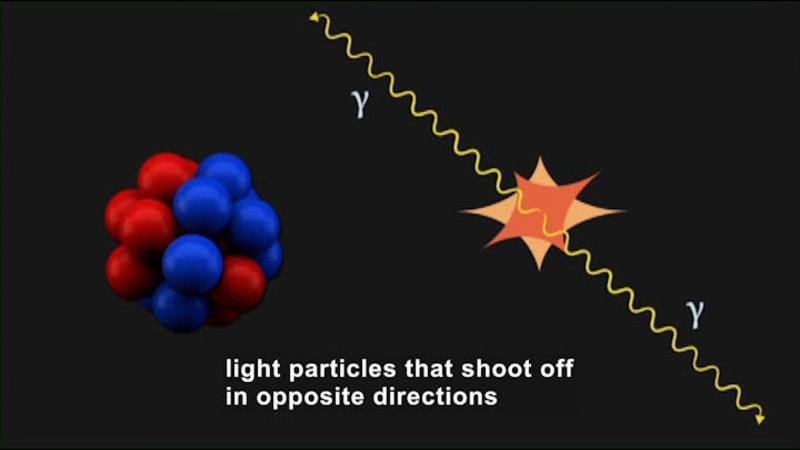
The rate of a chemical reaction is affected by a number of factors, including temperature and the concentration of reactants at the beginning of the reaction. While the chemical equation may show reactants turning into products as a straightforward process, it is actually involved and precise. How exactly do reactants turn into products? Sometimes, the answer is as simple as two atoms bumping into each other and forming a bond. Most of the time, however, the process is much more complex. Controlling the rate of reactions has implications for a variety of applications, including drug design and corrosion prevention. Part of the series Chemistry: Challenges And Solutions.
(Source: DCMP)
Metadata
- Subject:
- Physical Sciences - Science
- Keywords:
- conservation, electricity and magnetism, energy, physics
Files 1
-
Kinetics and Nuclear Chemistry: Rates Of Reaction

- Type:
- Video
- Format:
- Streaming
- Accommodations:
- English Audio Descriptions - Visual, English Captions - Auditory
- Languages:
- English
- License:
- GNU-GPL
